 NOVEL FOOD in EU
NOVEL FOOD in EU
In the EU, novel foods (NFs) are, according to Regulation (EU) 2015/2283, classified as products that have not been widely consumed in the EU prior to May 1997. These foodstuffs cannot be placed on the market before the authorisation by the European Commission (EC).
In order to place CBD products, which are classified as novel foods on the EU market, a business operator must submit an application to the EC for the authorisation of this particular product. The processing of the application is complex and consists of the following steps:
- Within the initial step, the European Commission (EC) and the European Food Safety Authority (EFSA) carry out a validity check of submitted NF application. Once all required information is submitted by the Applicant, the validation of NF application is confirmed.
- The following step is a risk assessment by the EFSA, which is usually requested by the EC. The EFSA expresses its opinion on the novel food within 9 months if there are not further requests from Authorities within this stage.
- Within 7 months of the EFSA’s opinion, the EC submits a draft implementing act, authorising the legal placement of the NF on the market.
- Once the act receives a favourable vote from the Standing Committee and is adopted by the EC, the NF can be lawfully placed on the EU market.
 NOVEL FOOD in UK
NOVEL FOOD in UK
The system of the NF authorisation is similar in the UK what regards legislation on NFs. However, when it comes to CBD products, the Food Standards Agency (FSA) decided to act a bit differently.
On the 13th of Feb 2020 the Authorities announced that businesses that sell CBD products in the UK need to submit and have a validated NF application by the 31st of March 2021. After this date, only products for which the FSA reviewed and assigned the corresponding application as valid are to be allowed to remain on the market.
This has changed in March 2021, when the FSA announced that businesses only need to submit the NF applications by the end of March 2021.
The FSA also established a so called “public list”, which provides public information on the Applicants which have their NF applications valid. The FSA clearly states that the process runs in parallel to the validation process of NF applications that are currently underway and does not impact the outcome of the validation status.
At the moment, all businesses can sell their CBD products in the UK, provided they are not incorrectly labelled, are not unsafe and are covered in their corresponding NF applications which were submitted prior to March 2021.
PHARMAHEMP – STATUS OF NOVEL FOOD APPLICATIONS
We are in the process of authorisation of two novel food ingredients, namely cannabidiol (CBD) and Cannabis sativa L. extract. Both applications were submitted to EC in the EU and to FSA in the UK in 2020 and 2021.
EC current status:
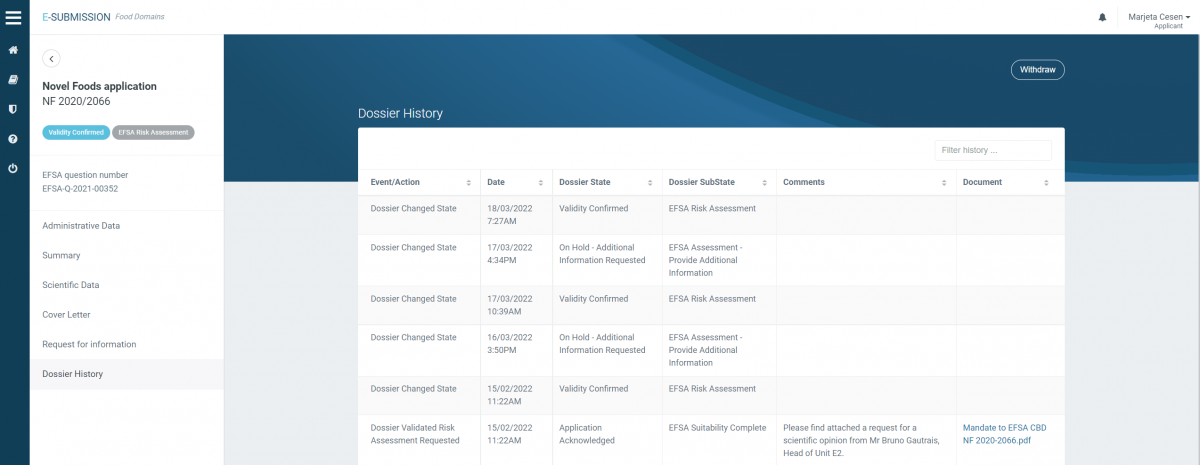
Novel food application for CBD (2020/2066) = VALID since February 2022
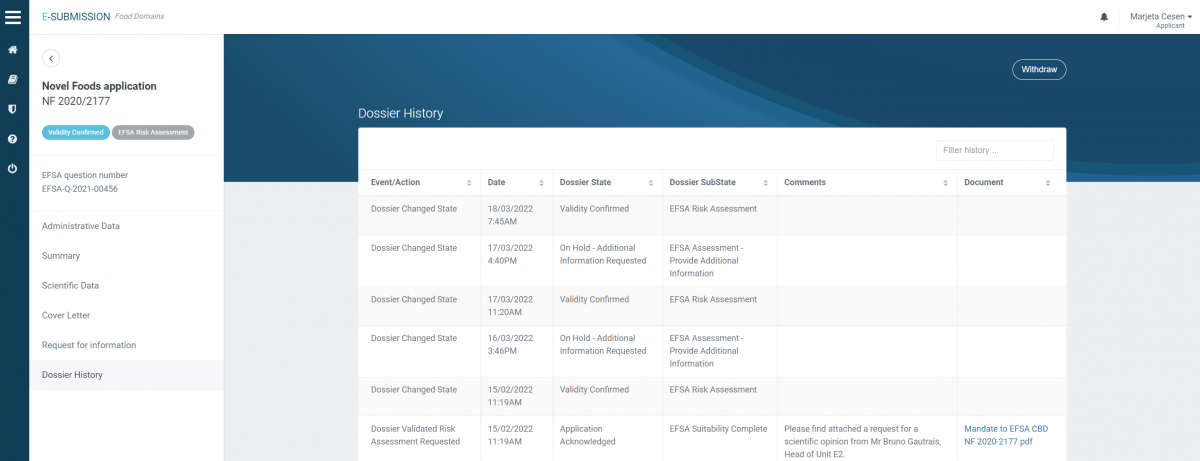
Novel food application for Cannabis sativa L. extract NF (2020/2177) = VALID since February 2022
The status of novel food applications is also publicly available on OpenEFSA Portal.
UK current status:
Novel food application for Cannabis sativa L. extract (RP-821) = validated (in the risk assessment stage)
545 products that are related to PharmaHemps’ NF application RP-821 are listed on the CBD Public list created by the Food Standards Agency.










